During the late 19th century, chemists developed the periodic table to organize chemical knowledge. However, when radioactivity was discovered, it seemed to contradict the periodic system. Samples with similar chemical behavior were found to have distinct physical properties such as half-life, emitted radiation type, and energy.
In 1913, the concept of isotopes was introduced by Soddy, which described elements occupying the "same place" in the periodic table, yet differing in their nuclear properties. However, a complete explanation awaited the discovery of the neutron by Chadwick in 1932.
Isotopes, Isobars, and Isotones
Several terms describe different relationships between protons and neutrons:
- Isotopes: Same number of protons (Z = constant).
- Isotones: Same number of neutrons (N = constant).
- Isobars: Same mass number (A = constant).
The term "isotope" is the most widely used. It is essential to specify the element or group of elements when discussing isotopes, such as ^{11}C, ^{12}C, ^{13}C, and ^{14}C as isotopes of carbon.
Nuclide Chart and Stability Line
The Nuclide Chart (Fig. 4.1) illustrates the stable nuclides along a line known as the stability line. The stability of a nucleus depends on two competing forces:
- The Strong Force: This binds protons and neutrons together.
- The Coulomb Force: This force repels particles with like charge (e.g., protons).
The balance between these forces is visualized in the chart, where the most stable nuclei lie along the stability line.
For optimal stability, the number of protons (\( Z \)) and neutrons (\( N \)) must be equal for lighter elements. This relationship can be represented as:
\( Z = N \) for light elements
For heavier elements, the stability line deviates due to the increasing Coulomb force. To counteract this force, additional neutrons are required, leading to an excess of neutrons. This deviation can be illustrated as:
\( N > Z \) for heavier elements
Understanding Nuclear Stability
The stability of the nucleus is a result of the interplay between the strong nuclear force and the Coulomb force. The stability line is nearly a straight line for light elements, but for heavier elements, the number of neutrons exceeds the number of protons. This is due to the larger Coulomb force between protons, which is mitigated by additional neutrons to maintain stability.
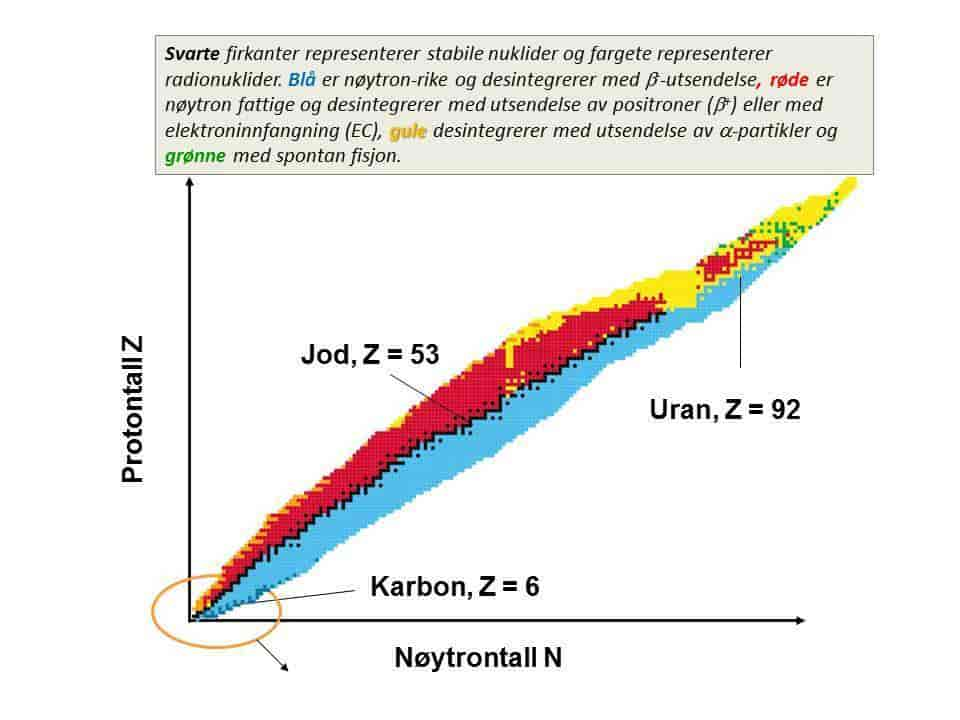
Figure 4.1: Nuclide Chart showing the stability line.
Conclusion
The concept of isotopes, isotones, and isobars, along with the understanding of the stability line on the nuclide chart, provides crucial insights into nuclear physics. The balance between the strong force and the Coulomb force dictates the stability of nuclei and their behavior in nuclear reactions.